CD SKILLS - OPTIMIZATION OF DIAGNOSTIC STRATEGIES IN CELIAC DISEASE (WPT2)
15-06-2022
Work package two “Optimization of diagnostic strategies in Celiac disease« aims to improve the diagnostics of celiac disease in the Danube region by harmonizing serum celiac antibody detection tools and optimize new technical approaches.
During the project we purified IgA patient antibodies to get reference material for calibration antibodies and developed cloned antibody preparates to transglutaminase epitopes that can be applied in defined concentrations in the assay as calibrators. Further, medical partners collected patient serum samples which represent panels of study material for comparison in each antibody measurement assay utilized by the different centers.
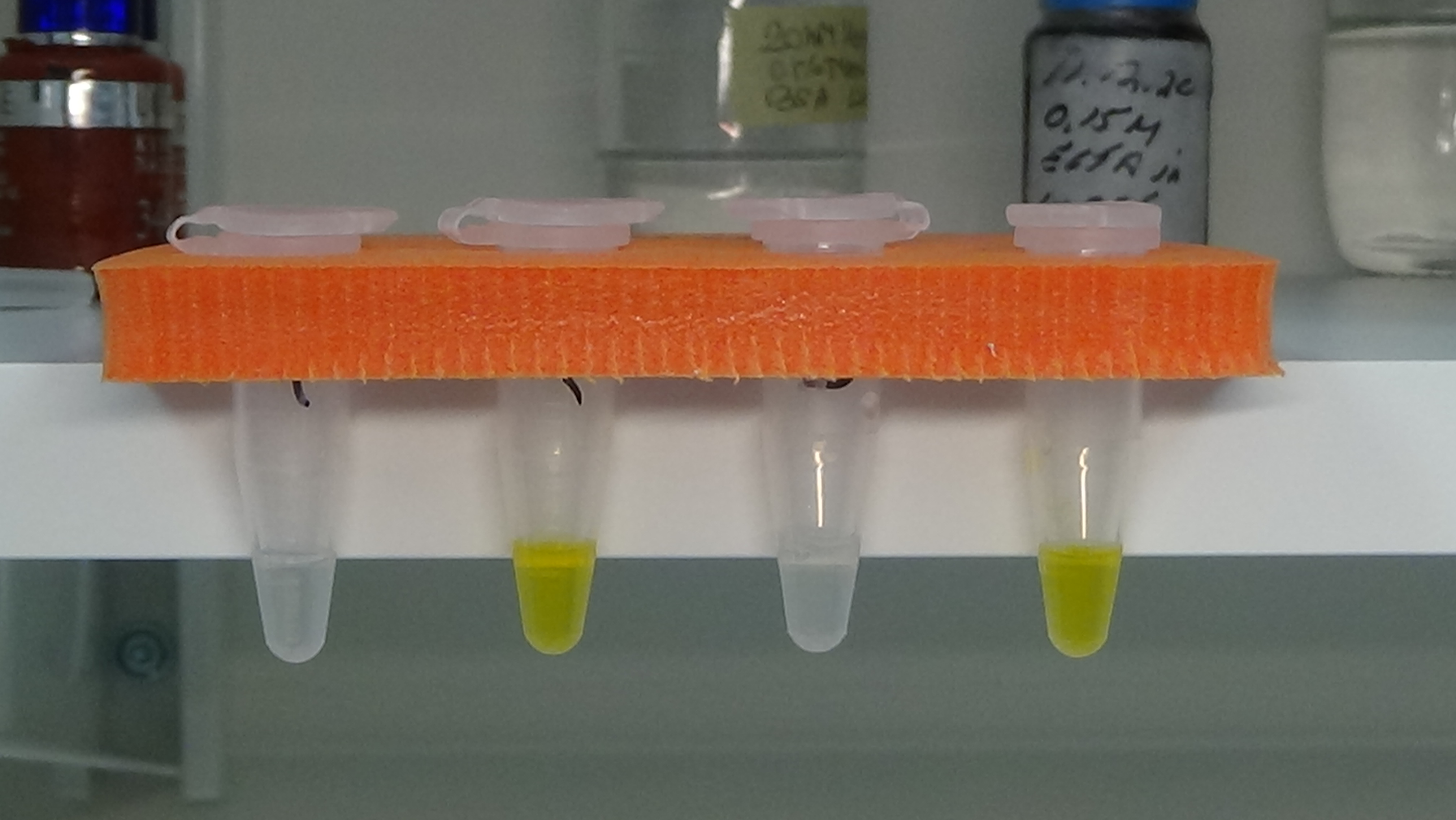
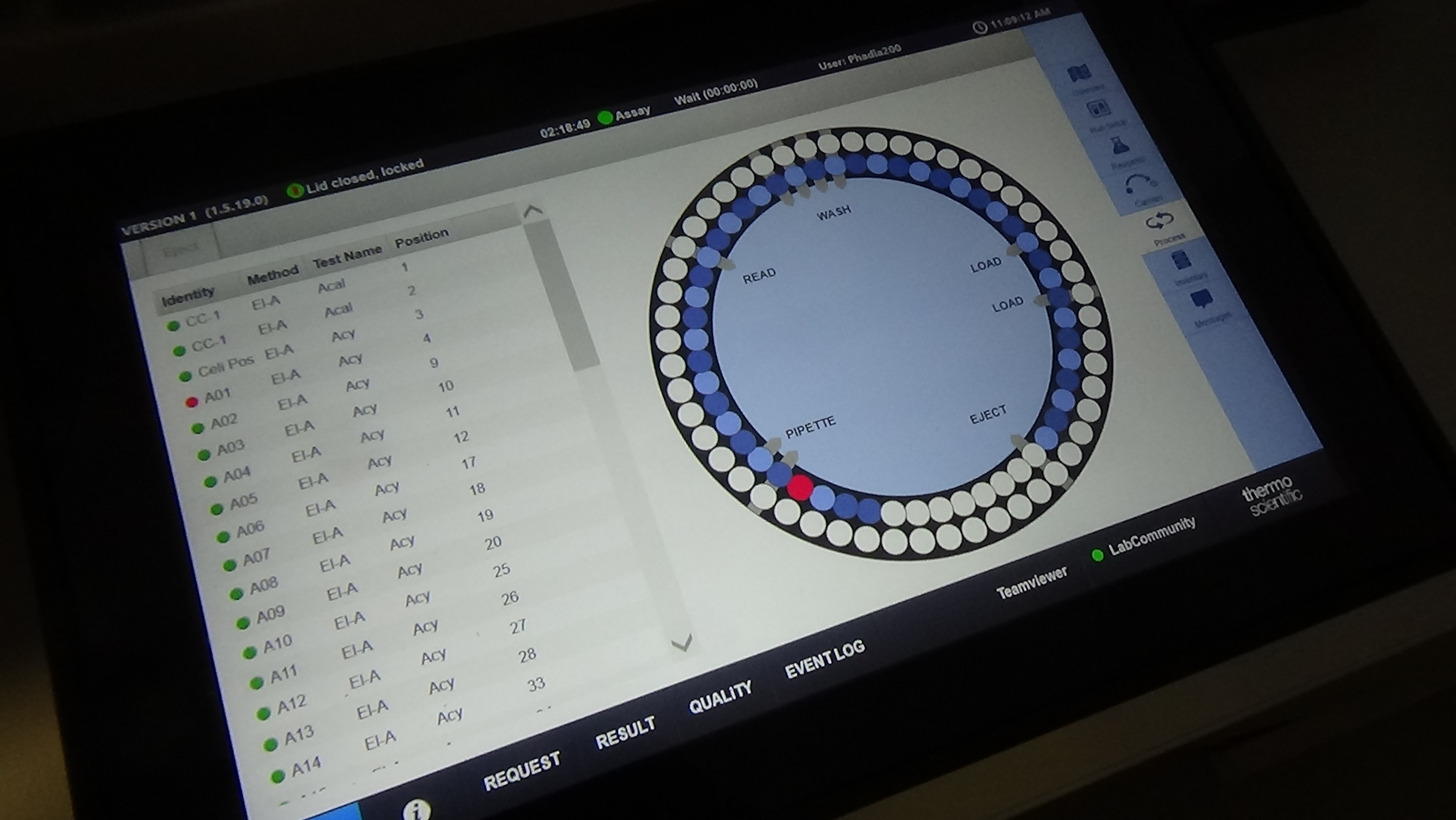
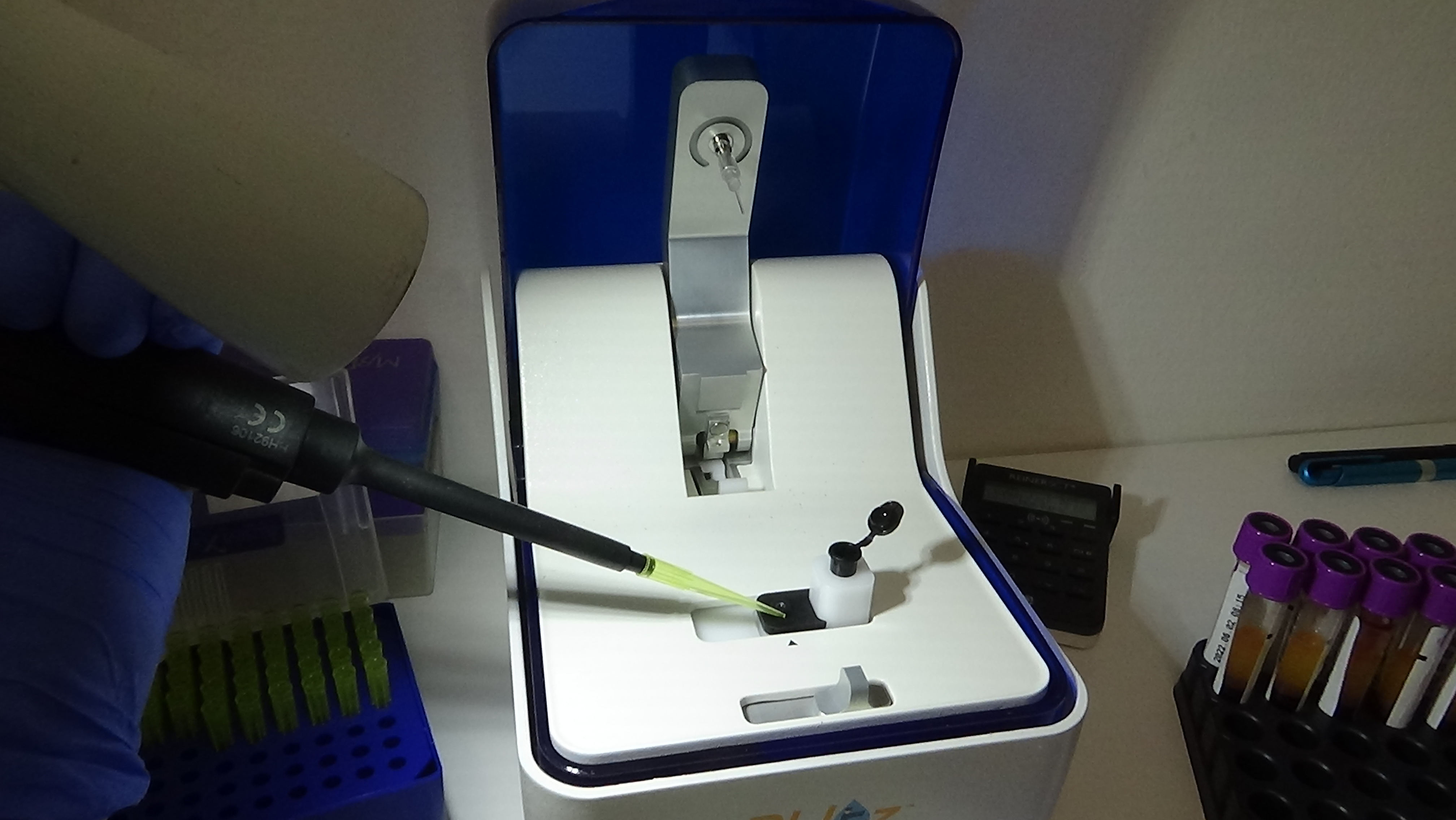
Approximately 320 serum samples have been collected so far by the project partners and one aliquot of each was sent in May t0 PP10 for central testing. Celiac disease diagnostic tests usually measure IgA class antibodies, since they are of the relevant immunoglobulin type for the disease process. In order to do so, enzyme-labelled secondary antibodies against human IgA are applied in the diagnostic assays. Upon binding, the enzyme component of these reagents generates an amplified colour or fluorescent signal which can be easily measured. When utilizing cloned antibodies as calibrators which are not of IgA type, signal generation can be ensured by conjugating the calibrator antibody directly to the signal producing enzyme. There are variations among different commercial celiac antibody tests which type of enzyme is used. Most use horse-radish peroxidase and we obtained good results with such type of conjugates on various commercial ELISA-based manual assays. However, newer celiac antibody tests are also run on automated, machine-based platforms where other enzymes are applied. One experimental antibody conjugation was tried with one of such enzymes and we were able to obtain an encouraging proof-of-concept preliminary result with it on the Phadia 200 platform. Another option is to introduce label-free measurement assays based on the bio-layer interferometry device (Blitz) purchased earlier for this project. Measurement optimisation with calibrators is in progress also on this platform.